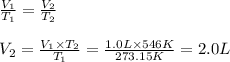Chemistry, 04.11.2020 19:00 kawaunmartinjr10
The volume of a sample of a gas is 1.0 liter at STP. If the pressure remains constant and the temperature is raised to 546 K, the new volume of the gas will be

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 11:20
The chemical composition of soil varies with depth. an article in communications in soil science and plant analysis describes chemical analyses of soil taken from a farm in western australia. fifty specimens were each taken at depths 50 and 250 cm. at a depth of 50 cm, the average no3 concentration (in mg/l) was 88.5 with a standard deviation of 49.4. at a depth of 250 cm, the average concentration was 110.6 with a standard deviation of 51.5. find a 95% confidence interval for the difference in no3 concentrations at the two depths.
Answers: 1

Chemistry, 23.06.2019 19:30
⁉️how many kj of energy would be needed to convert 150. g of ammonia to vapor at its boiling point? ⁉️(ammonia’s heat of vaporization is 1.38 kj/g
Answers: 3

Chemistry, 23.06.2019 22:00
Calculate the partial pressure, in atmospheres, of o2 in the dry air outside an airliner cruising at an altitude of about 20000 ft (6096 m), where the atmoshperic pressure is 351 mm hg. how much must the outside air be compressed to produce a cabin pressure in which the partial pressure of o2 is 0.200 atm?
Answers: 2
You know the right answer?
The volume of a sample of a gas is 1.0 liter at STP. If the pressure remains constant and the temper...
Questions

English, 02.08.2019 01:50






History, 02.08.2019 01:50



History, 02.08.2019 01:50


History, 02.08.2019 01:50

Mathematics, 02.08.2019 01:50



Mathematics, 02.08.2019 01:50

Spanish, 02.08.2019 01:50

Biology, 02.08.2019 01:50