Which model below represents an atom with an atomic mass of 4 units?
...

Chemistry, 04.11.2020 22:50 fonsworth5
Which model below represents an atom with an atomic mass of 4 units?
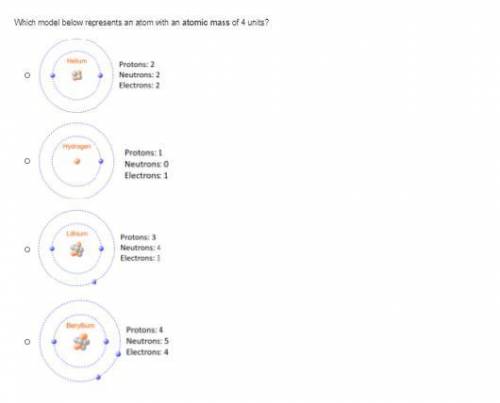

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Questions

Social Studies, 02.03.2021 22:10

Mathematics, 02.03.2021 22:10


English, 02.03.2021 22:10

Mathematics, 02.03.2021 22:10

Mathematics, 02.03.2021 22:10

Mathematics, 02.03.2021 22:10

Computers and Technology, 02.03.2021 22:10


History, 02.03.2021 22:10

Computers and Technology, 02.03.2021 22:10

Mathematics, 02.03.2021 22:10

Mathematics, 02.03.2021 22:10

History, 02.03.2021 22:10


English, 02.03.2021 22:10


Mathematics, 02.03.2021 22:10

Mathematics, 02.03.2021 22:10




