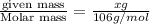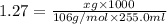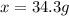Chemistry, 05.11.2020 18:30 jjjoooorrrrddddaannn
A chemist adds 255.0mL of a 1.27M sodium carbonate Na2CO3 solution to a reaction flask. Calculate the mass in grams of sodium carbonate the chemist has added to the flask.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3
You know the right answer?
A chemist adds 255.0mL of a 1.27M sodium carbonate Na2CO3 solution to a reaction flask. Calculate th...
Questions

Social Studies, 07.12.2020 03:30

Social Studies, 07.12.2020 03:30

Mathematics, 07.12.2020 03:30




Mathematics, 07.12.2020 03:30


English, 07.12.2020 03:30

Mathematics, 07.12.2020 03:30




Mathematics, 07.12.2020 03:30




Mathematics, 07.12.2020 03:30


Mathematics, 07.12.2020 03:30

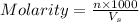
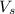 = volume of solution in ml
= volume of solution in ml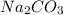 =
=