

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
4.0 moles of NaOH, a strong base, will completely dissociate in water. How many total moles of ions...
Questions

History, 25.08.2019 04:30

History, 25.08.2019 04:30


Physics, 25.08.2019 04:30


Mathematics, 25.08.2019 04:30

Chemistry, 25.08.2019 04:30


Mathematics, 25.08.2019 04:30

Geography, 25.08.2019 04:30

Physics, 25.08.2019 04:30

History, 25.08.2019 04:30


Social Studies, 25.08.2019 04:30



Mathematics, 25.08.2019 04:30

Physics, 25.08.2019 04:30

Geography, 25.08.2019 04:30

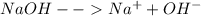
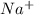 and 1 mole
and 1 mole 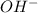 .
.

