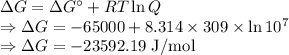Calculate the standard biological Gibbs free energy for the reaction: pyruvate- + NADH + H+(aq) ---> Lactate- + NAD+ at 309 K given that the standard Gibbs free energy = -65.0 kJ/mol at this temperature. This reaction occurs under conditions of low oxygen supply, such as in muscle cells during strenuous exercise. Note: See Box 7.1 on page 164. The biological standard state has hydrogen ions at 1x10-7 molar instead of 1 M.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2


Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

You know the right answer?
Calculate the standard biological Gibbs free energy for the reaction: pyruvate- + NADH + H+(aq) ---&...
Questions



Advanced Placement (AP), 17.12.2020 04:30




Physics, 17.12.2020 04:30


Arts, 17.12.2020 04:30

Geography, 17.12.2020 04:30

Mathematics, 17.12.2020 04:30


Mathematics, 17.12.2020 04:30




Mathematics, 17.12.2020 04:30

Chemistry, 17.12.2020 04:30


Biology, 17.12.2020 04:30

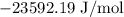
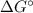 = Standard Gibbs free energy = -65.0 kJ/mol
= Standard Gibbs free energy = -65.0 kJ/mol![[H^+]](/tpl/images/0870/7831/07acb.png) = Biological standard state has hydrogen ions =
= Biological standard state has hydrogen ions = 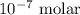
![Q=\dfrac{1}{[H^+]}\\\Rightarrow Q=\dfrac{1}{10^{-7}}\\\Rightarrow Q=10^7](/tpl/images/0870/7831/5fb93.png)