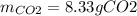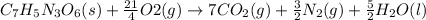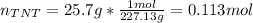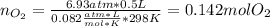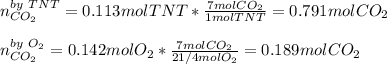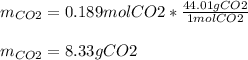Trinitrotoluene (TNT, C7H5N3O6) undergoes complete combustion according to the following balanced chemical equation:
C7H5N3O6(s)+214O2(g)→7CO2(g)+32N2(g )+52H2O(l)
If 25.7 g of TNT is combusted in a 0.500 L container filled with O2 at a pressure of 7.02 bar and a temperature of 298 K, calculate the maximum mass of CO2 that could be produced.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
Trinitrotoluene (TNT, C7H5N3O6) undergoes complete combustion according to the following balanced ch...
Questions



Mathematics, 03.04.2020 21:51

Social Studies, 03.04.2020 21:51


Mathematics, 03.04.2020 21:51






Mathematics, 03.04.2020 21:51




Mathematics, 03.04.2020 21:52

English, 03.04.2020 21:52

Mathematics, 03.04.2020 21:53


Mathematics, 03.04.2020 21:53