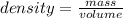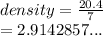Chemistry, 05.11.2020 22:00 dyllanmasters99
A student needs to determine the density of an unknown rock sample by dropping the rock into a graduated cylinder containing water. The original volume in the graduated cylinder is 23.0 ml. The student then drops the rock sample into the graduated cylinder and determines the new volume to be 30.0 ml. If the mass of the rock is determined to be 20.4 grams, what is the density of this sample?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 12:30
If you reacted 450 g of trimethylgallium with 300 g of arsine, what mass of gaas could you make?
Answers: 1

Chemistry, 23.06.2019 13:00
Using the periodic table complete the table to describe each atom type in your answers
Answers: 1
You know the right answer?
A student needs to determine the density of an unknown rock sample by dropping the rock into a gradu...
Questions

Social Studies, 25.08.2019 00:20






Arts, 25.08.2019 00:30

Biology, 25.08.2019 00:30

Mathematics, 25.08.2019 00:30



History, 25.08.2019 00:30



Mathematics, 25.08.2019 00:30


Mathematics, 25.08.2019 00:30

History, 25.08.2019 00:30


Mathematics, 25.08.2019 00:30