PLEASE HELP ASAP
The electron configuration of an element
shown below.
1s22s22p
N...

Chemistry, 06.11.2020 01:40 tricklts15
PLEASE HELP ASAP
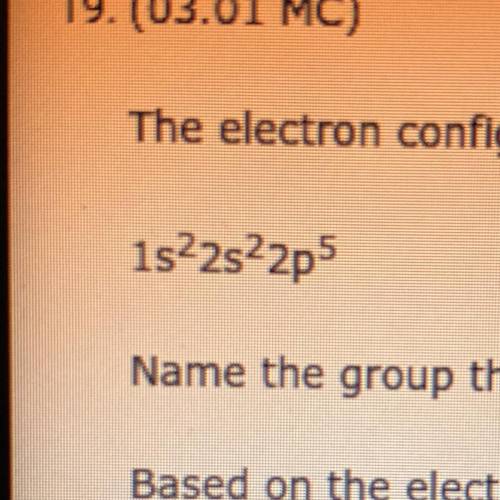
The electron configuration of an element
shown below.
1s22s22p
Name the group this element belongs to in the periodic table and explain your answer.
Based on the electron configuration, explain how many electrons it is likely to receive from the atom of another element during the formation of a bond.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

You know the right answer?
Questions

Arts, 28.10.2020 20:40


Biology, 28.10.2020 20:40


Mathematics, 28.10.2020 20:40

Mathematics, 28.10.2020 20:40


Mathematics, 28.10.2020 20:40


Mathematics, 28.10.2020 20:40




Biology, 28.10.2020 20:40


Mathematics, 28.10.2020 20:40




Mathematics, 28.10.2020 20:40



