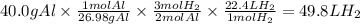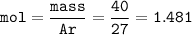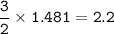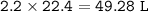Chemistry, 06.11.2020 04:20 crawford184232323234
What is the volume in liters of hydrogen gas that would be produced by the reaction of 40.0 g of Al with excess HCl at STP according to the following reaction? 2 Al (s) + 6 HCl (aq) → 2 AlCl₃ (aq) + 3 H₂ (g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2


Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
What is the volume in liters of hydrogen gas that would be produced by the reaction of 40.0 g of Al...
Questions

Mathematics, 20.05.2020 03:59

Mathematics, 20.05.2020 03:59

Mathematics, 20.05.2020 03:59

English, 20.05.2020 03:59

Mathematics, 20.05.2020 03:59


History, 20.05.2020 03:59

History, 20.05.2020 03:59

Biology, 20.05.2020 03:59

Social Studies, 20.05.2020 03:59

Mathematics, 20.05.2020 03:59


Health, 20.05.2020 04:00

Mathematics, 20.05.2020 04:00





Mathematics, 20.05.2020 04:00