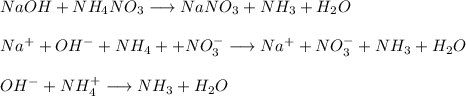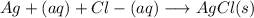Chemistry, 06.11.2020 16:20 toddbecca9
g Mark the statements as True or False. (1) When mixing, NaOH(aq) and NH4NO3 (aq) can not complete their reaction because there is no precipitate formation. (2) To quantify unknown soluble chloride, we could use either gravimetry or volumetric analysis based on AgCl(ppt) formation.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

You know the right answer?
g Mark the statements as True or False. (1) When mixing, NaOH(aq) and NH4NO3 (aq) can not complete t...
Questions


Chemistry, 30.11.2020 22:10

Mathematics, 30.11.2020 22:10

Chemistry, 30.11.2020 22:10


Mathematics, 30.11.2020 22:10

English, 30.11.2020 22:10



Advanced Placement (AP), 30.11.2020 22:10






Mathematics, 30.11.2020 22:10




Biology, 30.11.2020 22:20