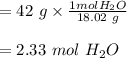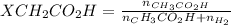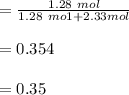Chemistry, 06.11.2020 17:10 nicolehathaway1012
A solution is made by mixing of water and of acetic acid . Calculate the mole fraction of water in this solution. Be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1


Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1
You know the right answer?
A solution is made by mixing of water and of acetic acid . Calculate the mole fraction of water in t...
Questions

History, 27.01.2021 15:00

History, 27.01.2021 15:00

Mathematics, 27.01.2021 15:00

Mathematics, 27.01.2021 15:00

Mathematics, 27.01.2021 15:00

Mathematics, 27.01.2021 15:00


Law, 27.01.2021 15:00


English, 27.01.2021 15:00


Mathematics, 27.01.2021 15:00

Mathematics, 27.01.2021 15:00

Mathematics, 27.01.2021 15:00


History, 27.01.2021 15:00

Mathematics, 27.01.2021 15:00

Mathematics, 27.01.2021 15:00


Mathematics, 27.01.2021 15:00

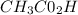
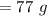
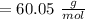
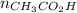
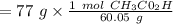
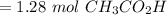
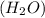
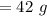
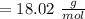
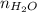 :
: