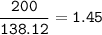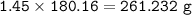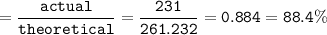PLEASE HELP I HAVE NO IDEA WHAT TO DO
Use the balanced equation below to answer the question. A student begins a reaction with 200.0 grams of C7H603. Calculate the percent yield of the reaction if 231.0 grams of C9H304 is actually
produced.
C7H. O3 + C4H8O3 C9H204 + C2H4O2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
PLEASE HELP I HAVE NO IDEA WHAT TO DO
Use the balanced equation below to answer the question. A stu...
Questions




Computers and Technology, 16.02.2021 05:40



Mathematics, 16.02.2021 05:40


Advanced Placement (AP), 16.02.2021 05:40


Chemistry, 16.02.2021 05:40



Computers and Technology, 16.02.2021 05:40




Mathematics, 16.02.2021 05:40

Social Studies, 16.02.2021 05:40