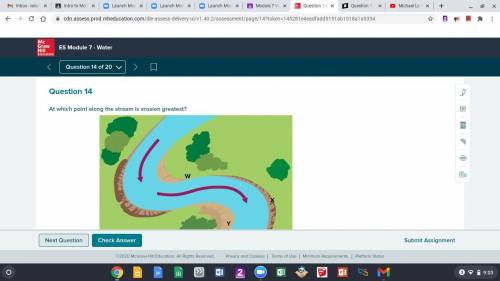
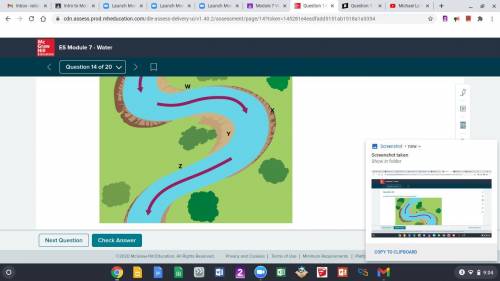
HELP!
A. W
B. X
C. Y
D. Z
...

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1

Chemistry, 23.06.2019 11:50
Achemist needs to prepare a buffer solution of ph 8.80. what molarity of nh3 (pkb = 4.75) is required to produce the buffer solution if the (nh4)2so4 in the solution is 1.8 m?
Answers: 1

Chemistry, 23.06.2019 12:30
Choose one literary selection from this semester in which you think the setting has a great impact on the work. in a full paragraph name the work, describe the setting, and explain why it is so important to the overall story or poem.
Answers: 1
You know the right answer?
Questions


Mathematics, 12.10.2020 21:01

History, 12.10.2020 21:01


History, 12.10.2020 21:01

History, 12.10.2020 21:01



English, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01


Mathematics, 12.10.2020 21:01


Chemistry, 12.10.2020 21:01


Mathematics, 12.10.2020 21:01

Social Studies, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

Biology, 12.10.2020 21:01