
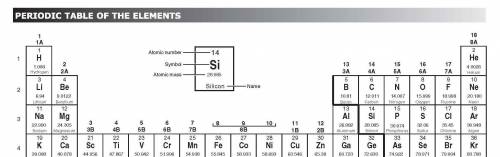
How would an element on the left side of row 2 of the periodic table differ from an element in the middle of the same row?
A. The element on the left would have more atomic mass.
B. The element on the left would have less malleability.
C. The element on the left would have a lower melting point.
D. The element on the left would have no metallic properties.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3
You know the right answer?
How would an element on the left side of row 2 of the periodic table differ from an element in the m...
Questions






Mathematics, 19.05.2020 03:16



History, 19.05.2020 03:17

English, 19.05.2020 03:17

Mathematics, 19.05.2020 03:17

Mathematics, 19.05.2020 03:17


Mathematics, 19.05.2020 03:17

Mathematics, 19.05.2020 03:17

Mathematics, 19.05.2020 03:17


Mathematics, 19.05.2020 03:17


History, 19.05.2020 03:17



