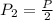Chemistry, 06.11.2020 22:50 davidswafforddd478
Boyles Law P1V1 = P2V2
An arrangement of helium balloons in the back of a delivery truck travels from a town at the base of a mountain up the mountain where the air pressure is half the amount. What do you expect to happen to the balloons?
A. The balloons will increase to twice their original volume.
B. The balloons will decease to one fourth their original size.
.C. The balloons will increase to four times their original size.
D. The balloons will decrease to half their original size.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
Boyles Law P1V1 = P2V2
An arrangement of helium balloons in the back of a delivery truck travels fr...
Questions

Mathematics, 03.07.2019 07:30

Mathematics, 03.07.2019 07:30





Chemistry, 03.07.2019 07:30


Chemistry, 03.07.2019 07:30

Mathematics, 03.07.2019 07:30





Mathematics, 03.07.2019 07:30

Mathematics, 03.07.2019 07:30




Business, 03.07.2019 07:30

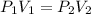
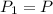 , initial volume be V, i.e.
, initial volume be V, i.e. 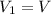 . The pressure is then halved, i.e.
. The pressure is then halved, i.e.