
Chemistry, 06.11.2020 23:10 kokilavani
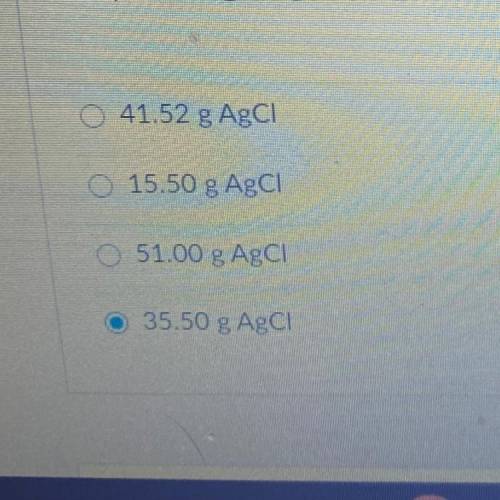
15.50 g of NH4Cl reacts with an excess of AgNO3. In the reaction 35.50 g
AgCl is produced. What is the percent yield of AgCl?
NH4Cl + AgNO3 --> AgCl + NH4NO3
69.61 % Yield AgCl
0 43.66 % Yield AgCl
85.50 % Yield AgCI
O 85.50 g AgCI

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 08:30
What percentage of energy used in the u.s is produced from fossil fuels
Answers: 2

Chemistry, 23.06.2019 09:30
My plate recommends that of your nutritional intake comes from fruits and vegetables. a. 30% b. 50% c. 20% d. 40%
Answers: 2
You know the right answer?
15.50 g of NH4Cl reacts with an excess of AgNO3. In the reaction 35.50 g
AgCl is produced. What is...
Questions

Mathematics, 05.05.2021 03:10

History, 05.05.2021 03:10

Health, 05.05.2021 03:10

Business, 05.05.2021 03:10


English, 05.05.2021 03:10

Mathematics, 05.05.2021 03:10


History, 05.05.2021 03:10

Mathematics, 05.05.2021 03:10

Mathematics, 05.05.2021 03:10

Mathematics, 05.05.2021 03:10



Mathematics, 05.05.2021 03:10



Biology, 05.05.2021 03:10

Mathematics, 05.05.2021 03:10



