
Chemistry, 07.11.2020 01:00 chaycebell6662
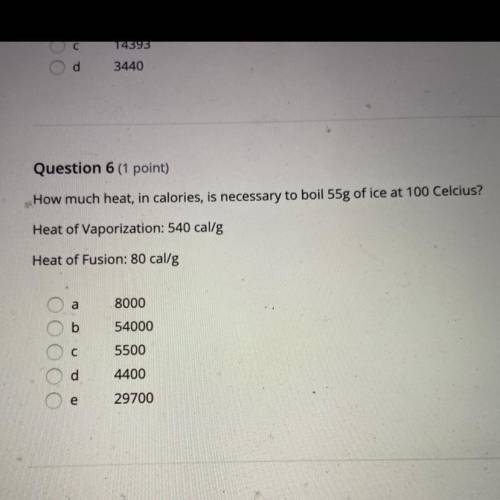
Question 6 (1 point)
How much heat, in calories, is necessary to boil 55g of ice at 100 Celcius?
Heat of Vaporization: 540 cal/g
Heat of Fusion: 80 cal/g
a
OO
С
8000
54000
5500
4400
29700
OC
e

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2
You know the right answer?
Question 6 (1 point)
How much heat, in calories, is necessary to boil 55g of ice at 100 Celcius?
Questions

Mathematics, 20.01.2020 15:31








Mathematics, 20.01.2020 15:31

Mathematics, 20.01.2020 15:31

Biology, 20.01.2020 15:31


Social Studies, 20.01.2020 15:31



Mathematics, 20.01.2020 15:31

English, 20.01.2020 15:31





