How do the resonance structures for ozone, O3, differ?
A. They have different numbers of atoms...

Chemistry, 07.11.2020 06:20 zulaykaalex
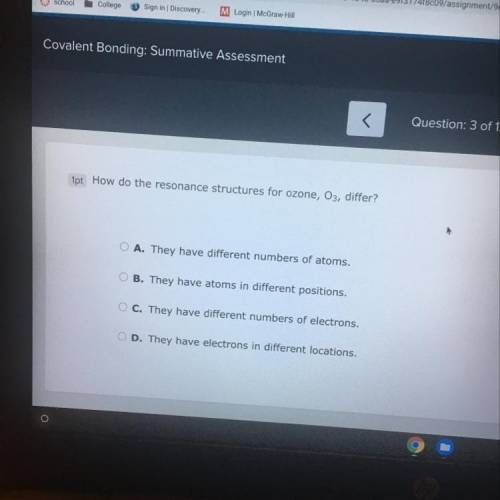
How do the resonance structures for ozone, O3, differ?
A. They have different numbers of atoms.
B. They have atoms in different positions,
C. They have different numbers of electrons.
D. They have electrons in different locations,

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
Questions

Mathematics, 14.12.2019 15:31



Mathematics, 14.12.2019 15:31



Computers and Technology, 14.12.2019 15:31

Mathematics, 14.12.2019 15:31

Social Studies, 14.12.2019 15:31

Health, 14.12.2019 15:31

History, 14.12.2019 15:31

English, 14.12.2019 15:31



Biology, 14.12.2019 15:31

Biology, 14.12.2019 15:31

English, 14.12.2019 15:31

Mathematics, 14.12.2019 15:31


History, 14.12.2019 15:31



