Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 23.06.2019 07:30
How many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1

Chemistry, 23.06.2019 09:30
People who practice which of the following diets may run the risk of not getting enough iron. a. gluten free or vegan diet b. diet for managing diabetes c. vegan diet d. gluten free diet
Answers: 2
You know the right answer?
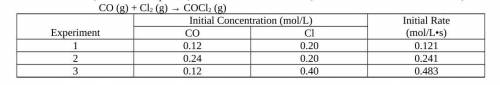
Using the experimental data provided, determine the order of reaction with respect to each reactant,...
Questions


Mathematics, 19.12.2020 05:20


Mathematics, 19.12.2020 05:20

English, 19.12.2020 05:20

History, 19.12.2020 05:20


Mathematics, 19.12.2020 05:20



Mathematics, 19.12.2020 05:20

Mathematics, 19.12.2020 05:20





Mathematics, 19.12.2020 05:20

Mathematics, 19.12.2020 05:20

Mathematics, 19.12.2020 05:20

Mathematics, 19.12.2020 05:20