
Chemistry, 08.11.2020 06:40 polyanskiymichael
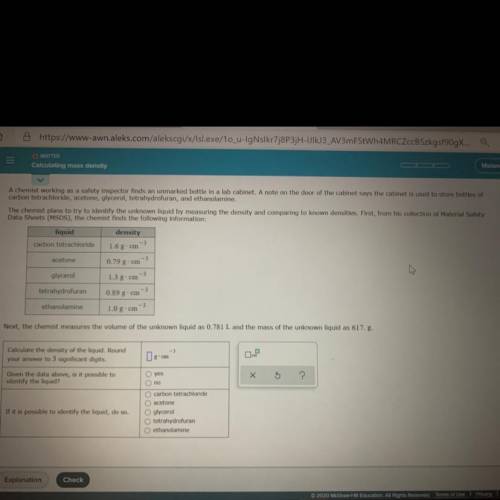
A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used
to store bottles of carbon tetrachloride, acetone, glycerol, tetrahydrofuran, and ethanolamine,
The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection
of Material Safety Data Sheets (MSDS), the chemist finds the following information:
liquid
density
carbon tetrachloride 1.6g. cm
ole
acetone
0,79 g" C11
glycerol
1.3 g cm
tetrahydrofuran
13
(0,89 g • CHI
ethanolamine
1.0 g. cm
Next, the chemist measures the volume of the unknown liquid as 0.781 L and the mass of the unknown liquid as 617. g.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the doo...
Questions

Chemistry, 04.01.2020 00:31








Mathematics, 04.01.2020 00:31




English, 04.01.2020 00:31

Mathematics, 04.01.2020 00:31






Social Studies, 04.01.2020 00:31



