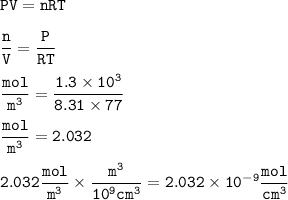Chemistry, 08.11.2020 08:20 jhjhgjvygv
vacuum line is lowered to a pressure of 1.3kpa and 77 k find the number of moles per mm3. Assume that R= 8.31 JK-1 mol-1

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
vacuum line is lowered to a pressure of 1.3kpa and 77 k find the number of moles per mm3. Assume tha...
Questions


Social Studies, 20.05.2021 21:20



Physics, 20.05.2021 21:20

Mathematics, 20.05.2021 21:20

Mathematics, 20.05.2021 21:20



Mathematics, 20.05.2021 21:20

Mathematics, 20.05.2021 21:20

Mathematics, 20.05.2021 21:20

Chemistry, 20.05.2021 21:20

Mathematics, 20.05.2021 21:20

Mathematics, 20.05.2021 21:20



Mathematics, 20.05.2021 21:20

Physics, 20.05.2021 21:20

Mathematics, 20.05.2021 21:20