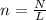Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 23.06.2019 08:30
What percentage of energy used in the u.s is produced from fossil fuels
Answers: 2
You know the right answer?
How many moles is 4.98x1028 molecules of NaBr?...
Questions

English, 17.12.2020 22:20

Arts, 17.12.2020 22:20

History, 17.12.2020 22:20


Mathematics, 17.12.2020 22:20

History, 17.12.2020 22:20

Law, 17.12.2020 22:20

Physics, 17.12.2020 22:20



Social Studies, 17.12.2020 22:20

Biology, 17.12.2020 22:20


Mathematics, 17.12.2020 22:20

Biology, 17.12.2020 22:20




Mathematics, 17.12.2020 22:20

Mathematics, 17.12.2020 22:20