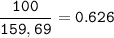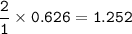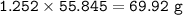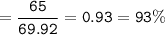Chemistry, 08.11.2020 16:40 athenajames1221
Iron is extracted from iron oxide in the Blast Furnace: Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2
a) Calculate the maximum theoretical mass of iron that can be made from 100g
of iron oxide.
b) In the reaction, only 65 g of iron was made. Calculate the percentage yield.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2



Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
Iron is extracted from iron oxide in the Blast Furnace: Fe 2 O 3 + 3 CO → 2 Fe + 3 CO 2
a) Calculat...
Questions






Chemistry, 16.10.2020 03:01


Mathematics, 16.10.2020 03:01

English, 16.10.2020 03:01

Biology, 16.10.2020 03:01



Mathematics, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01






Social Studies, 16.10.2020 03:01