
Chemistry, 09.11.2020 01:40 andrewcamp99
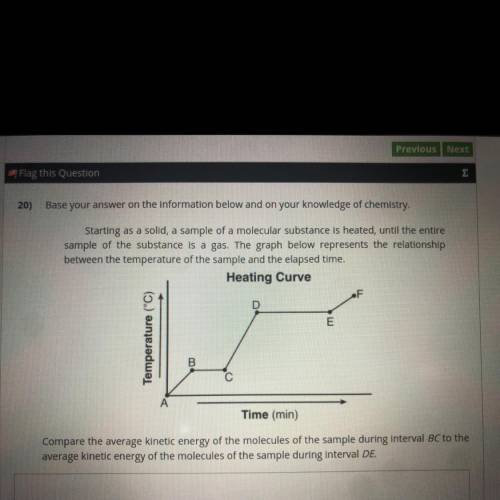
Starting as a solid, a sample of a molecular substance is heated, until the entire sample of the substance is a gas. The graph below represents the relationship
between the temperature of the sample and the elapsed time.
Temperature
Compare the average kinetic energy of the molecules of the sample during Interval BC to the
average kinetic energy of the molecules of the sample during interval DE.

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1

Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3

Chemistry, 23.06.2019 07:30
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3

Chemistry, 23.06.2019 10:30
Which of the following characteristics are true of enzymes? check all that apply. a.)the structure of an enzyme can change if conditions change. b.)a single enzyme can normally catalyze a wide variety of reactions under many conditions. c.)enzymes are found only in nonliving systems. d.)enzymes allow living things to regulate body conditions through feedback mechanisms. e.)enzymes bind to specific substrates in specific ways. f.)enzymes increase the rate of reaction. g.)when shown in energy-reaction diagrams, enzymes represent the higher activation energy.
Answers: 1
You know the right answer?
Starting as a solid, a sample of a molecular substance is heated, until the entire sample of the sub...
Questions

History, 01.02.2020 14:43


History, 01.02.2020 14:43

English, 01.02.2020 14:43

World Languages, 01.02.2020 14:43


Chemistry, 01.02.2020 14:43



Mathematics, 01.02.2020 14:43





Biology, 01.02.2020 14:43


History, 01.02.2020 14:43

Health, 01.02.2020 14:43

Chemistry, 01.02.2020 14:43



