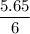Chemistry, 09.11.2020 01:50 Rocket3138
Calculate the heat evolved for the reaction of 2.50 L of B2H6 with 5.65 L of Cl2 according to the following reaction. Both reactant gases are initially at STP B2H6(g) + 6 Cl2(g) —> 3 BCl3(g) + 6HCl(g) Delta H = -1396 kJ

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 23.06.2019 05:30
What is the body’s main processing system? it uses input from various parts to control voluntary and involutiontary movement. it’s composed of two main parts-the brain and spinal cord. a. nbs b.cns c. ans d. pns
Answers: 1

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
Calculate the heat evolved for the reaction of 2.50 L of B2H6 with 5.65 L of Cl2 according to the fo...
Questions

Social Studies, 29.05.2020 03:01

Mathematics, 29.05.2020 03:01





Biology, 29.05.2020 03:01

History, 29.05.2020 03:01




English, 29.05.2020 03:01


History, 29.05.2020 03:01

Mathematics, 29.05.2020 03:01

English, 29.05.2020 03:01

Mathematics, 29.05.2020 03:01



Chemistry, 29.05.2020 03:01

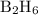 has been 58.637 kJ.
has been 58.637 kJ.