
Chemistry, 09.11.2020 01:50 tristenmathews
Consider the following reaction: 4HCl(g)+O2(g)→2H2O(g)+2Cl2(g) Each of the following molecular diagrams represents an initial mixture of the reactants.(Figure 1) How many molecules of Cl2 would be formed from the reaction mixture that produces the greatest amount of products?
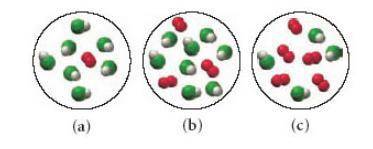

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
Consider the following reaction: 4HCl(g)+O2(g)→2H2O(g)+2Cl2(g) Each of the following molecular diagr...
Questions

Physics, 29.05.2020 21:04


English, 29.05.2020 21:04





Biology, 29.05.2020 21:04







History, 29.05.2020 21:04

Mathematics, 29.05.2020 21:04

Mathematics, 29.05.2020 21:04


Social Studies, 29.05.2020 21:04

Mathematics, 29.05.2020 21:04



