
Chemistry, 09.11.2020 05:10 jenellalexis94p5jp7i
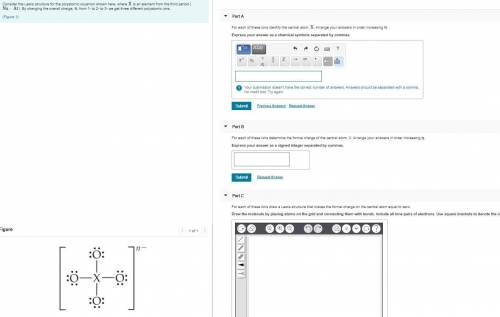
Consider the Lewis structure for the polyatomic oxyanion shown here, where X is an element from the third period (Na−Ar). By changing the overall charge, n, from 1- to 2- to 3- we get three different polyatomic ions.
a) For each of these ions identify the central atom, X. Arrange your answers in order increasing n. Express your answer as a chemical symbols separated by commas.
b) For each of these ions determine the formal charge of the central atom, X. Arrange your answers in order increasing n. Express your answer as a signed integer separated by commas.
c) For each of these ions draw a Lewis structure that makes the formal charge on the central atom equal to zero. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons. Use square brackets to denote the overall charge.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

You know the right answer?
Consider the Lewis structure for the polyatomic oxyanion shown here, where X is an element from the...
Questions

Biology, 04.11.2019 05:31


History, 04.11.2019 05:31




Geography, 04.11.2019 05:31



Chemistry, 04.11.2019 05:31

Mathematics, 04.11.2019 05:31




History, 04.11.2019 05:31




Mathematics, 04.11.2019 05:31



