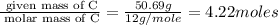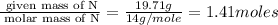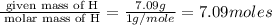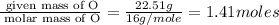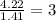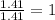Chemistry, 09.11.2020 17:00 maribelsalgado3
Polyacrylamide is used in manufacturing soft contact lenses. It consist of 50.69% carbon, 19.71% nitrogen, 7.09% hydrogen, and 22.51% oxygen. Calculate the empirical formula for polyacrylamide.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1

Chemistry, 23.06.2019 11:40
Which of the following is true for a reliable scientific source? it cites logic. it cites opinions. it cites valid data. it cites common sense.
Answers: 2
You know the right answer?
Polyacrylamide is used in manufacturing soft contact lenses. It consist of 50.69% carbon, 19.71% nit...
Questions

Mathematics, 09.04.2020 02:04




Biology, 09.04.2020 02:04

English, 09.04.2020 02:04

History, 09.04.2020 02:04






History, 09.04.2020 02:04




Mathematics, 09.04.2020 02:05

Mathematics, 09.04.2020 02:05


Mathematics, 09.04.2020 02:05

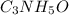 .
.