

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1
You know the right answer?
Find the percent composition of a compound that contains 2.63 g of carbon, 0.370 g of hydrogen and 0...
Questions

Mathematics, 26.02.2020 20:18





English, 26.02.2020 20:18





Chemistry, 26.02.2020 20:19

English, 26.02.2020 20:19

Physics, 26.02.2020 20:19







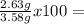 73.46 %Hydrogen:
73.46 %Hydrogen: 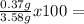 10.34 %Oxygen:
10.34 %Oxygen: 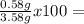 16.20 %
16.20 %

