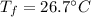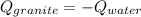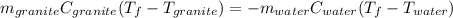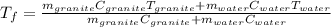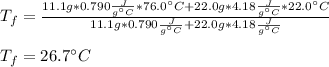Chemistry, 09.11.2020 17:20 alysonmariefont
A 11.1-g sample of granite initially at 76.0°C is immersed into 22.0 g of water initially at 22.0°C. What is the final temperature of both substances when they reach thermal equilibrium? (For water, Cs=4.18J/g⋅∘C and for granite, Cs=0.790J/g⋅∘C.)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
A 11.1-g sample of granite initially at 76.0°C is immersed into 22.0 g of water initially at 22.0°C....
Questions


Mathematics, 08.03.2020 01:01

Mathematics, 08.03.2020 01:01



English, 08.03.2020 01:02


Mathematics, 08.03.2020 01:02

Mathematics, 08.03.2020 01:02





Mathematics, 08.03.2020 01:03



Mathematics, 08.03.2020 01:04

Physics, 08.03.2020 01:04

Mathematics, 08.03.2020 01:04