
Chemistry, 09.11.2020 19:20 mariam00000w
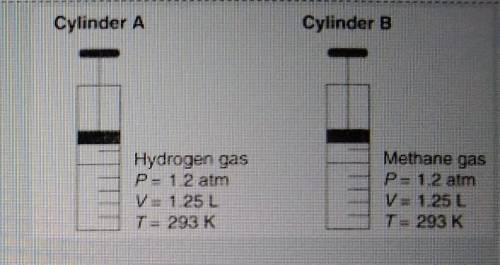
Diagram shows that both gases occupy the same volume under the same conditions of temperature and pressure. Show a numerical set up for how you will calculate the new volume of the gas, if the pressure remains constant ( at 1.2atm), but the temperature is raised from 293k to 398K.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

You know the right answer?
Diagram shows that both gases occupy the same volume under the same conditions of temperature and pr...
Questions

Mathematics, 27.01.2021 07:00

Business, 27.01.2021 07:00


Mathematics, 27.01.2021 07:00

Mathematics, 27.01.2021 07:00


History, 27.01.2021 07:00






Mathematics, 27.01.2021 07:00


History, 27.01.2021 07:00

History, 27.01.2021 07:00

Computers and Technology, 27.01.2021 07:00

Mathematics, 27.01.2021 07:00

Biology, 27.01.2021 07:00

Mathematics, 27.01.2021 07:00



