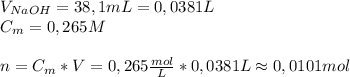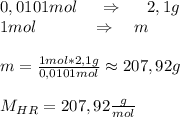Chemistry, 29.08.2019 10:10 JFrocks2480
A2.10g of unknown monoprotic acid is titrated with 38.10 ml of .265m naoh. calculate the molar mass of the ac? i already calculated that there are .0101 moles of naoh so that i have .0101 mol of the unknown acid.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1

Chemistry, 23.06.2019 21:30
This element is in the same family aa lead and it has fewer protons than sodium.
Answers: 2
You know the right answer?
A2.10g of unknown monoprotic acid is titrated with 38.10 ml of .265m naoh. calculate the molar mass...
Questions







Biology, 28.04.2021 03:00

Mathematics, 28.04.2021 03:00

Mathematics, 28.04.2021 03:00

Geography, 28.04.2021 03:00


English, 28.04.2021 03:00

Mathematics, 28.04.2021 03:00

Mathematics, 28.04.2021 03:00


History, 28.04.2021 03:00

Computers and Technology, 28.04.2021 03:00


Mathematics, 28.04.2021 03:00

Mathematics, 28.04.2021 03:00