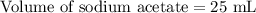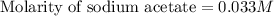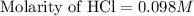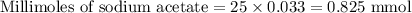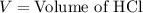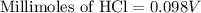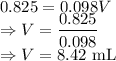Chemistry, 09.11.2020 22:50 deanlmartin
A 25.00 ml solution containing 0.033 M sodium acetate is titrated with a 0.098 M solution of HCl. How mar milliliters of HCl are required to reach the endpoint?

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
A 25.00 ml solution containing 0.033 M sodium acetate is titrated with a 0.098 M solution of HCl. Ho...
Questions




Biology, 28.10.2020 18:20

Chemistry, 28.10.2020 18:20


Social Studies, 28.10.2020 18:20

Physics, 28.10.2020 18:20


Mathematics, 28.10.2020 18:20





Mathematics, 28.10.2020 18:20

History, 28.10.2020 18:20

Mathematics, 28.10.2020 18:20