
Chemistry, 10.11.2020 01:30 zachstonemoreau
Iron is extracted from its ore using carbon:
2 Fe203 + 3C --> 4Fe + 3 CO2,
What is the atom economy of this reaction
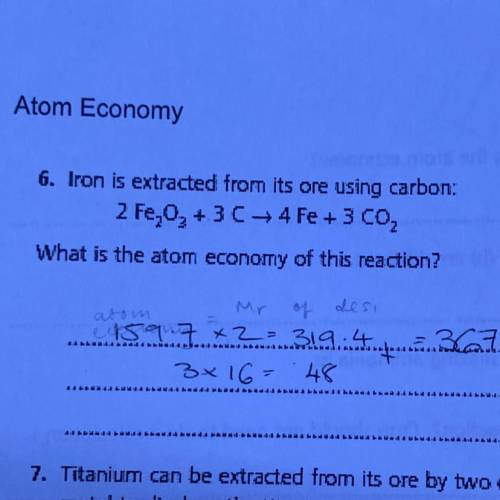

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2
You know the right answer?
Iron is extracted from its ore using carbon:
2 Fe203 + 3C --> 4Fe + 3 CO2,
What is the ato...
What is the ato...
Questions

Mathematics, 13.07.2021 17:30




English, 13.07.2021 17:30



Mathematics, 13.07.2021 17:30






Computers and Technology, 13.07.2021 17:30





Computers and Technology, 13.07.2021 17:30



