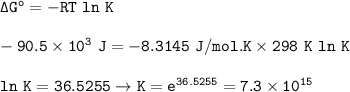+
Determine the equilibrium constant for the following reaction at 298 K. SO3
+ H20
H2S...

Chemistry, 10.11.2020 19:50 elijahdecent6070
+
Determine the equilibrium constant for the following reaction at 298 K. SO3
+ H20
H2SO4 (1)
AG° = -90.5 kJ
O a. 1.37 X 10-16
O b.4.78 X 10+11
O c. 9.11 X 10-8
O d. 7.31 X 10+15

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
Questions


Mathematics, 30.09.2019 21:40

Business, 30.09.2019 21:40

Mathematics, 30.09.2019 21:40


Mathematics, 30.09.2019 21:40


History, 30.09.2019 21:40

Mathematics, 30.09.2019 21:40



Advanced Placement (AP), 30.09.2019 21:40



Mathematics, 30.09.2019 21:40




Mathematics, 30.09.2019 21:40

English, 30.09.2019 21:40