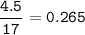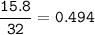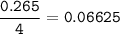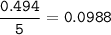Chemistry, 11.11.2020 06:10 sofyan00404
The chemical equation represents a reaction between ammonia and oxygen to form nitrogen monoxide and water. 4 N H 3 ( g ) + 5 O 2 ( g ) → 4 NO ( g ) + 6 H 2 O ( g ) What is the limiting reagent when a 4.50 g sample of ammonia is mixed with 15.80 g of oxygen?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2


Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
The chemical equation represents a reaction between ammonia and oxygen to form nitrogen monoxide and...
Questions

Mathematics, 06.05.2021 21:30

Mathematics, 06.05.2021 21:30




English, 06.05.2021 21:30

Mathematics, 06.05.2021 21:30


Mathematics, 06.05.2021 21:30



Chemistry, 06.05.2021 21:30

Mathematics, 06.05.2021 21:30

Mathematics, 06.05.2021 21:30

Arts, 06.05.2021 21:30

English, 06.05.2021 21:30




Mathematics, 06.05.2021 21:30