
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
(attached below)
response, do the following:
• Label all parts (1–9), including the solutions in each beaker and the connecting tube.
• Label which cell is the cathode and which cell is the anode. Include the charge on each strip.
• Show, or describe in detail, the flow of electrons.
• Describe what type of electrochemical cell is pictured. Explain how the cell works. Include the oxidation and reduction half-reactions in your explanation.
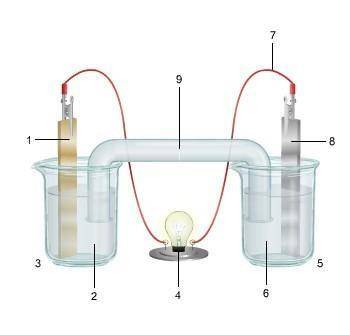

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1


Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
You know the right answer?
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
(attached...
Questions

Mathematics, 19.01.2020 00:31

Biology, 19.01.2020 00:31

Advanced Placement (AP), 19.01.2020 00:31

Mathematics, 19.01.2020 00:31

Mathematics, 19.01.2020 00:31

History, 19.01.2020 00:31





Mathematics, 19.01.2020 00:31


Mathematics, 19.01.2020 00:31

English, 19.01.2020 00:31

Mathematics, 19.01.2020 00:31







