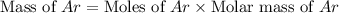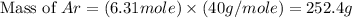Chemistry, 19.01.2020 22:31 Jtblack2720
What is the mass of 3.8 × 10^24 atoms of argon (ar)? 0.0039 g 0.16 g 6.3 g 250 g

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
What is the mass of 3.8 × 10^24 atoms of argon (ar)? 0.0039 g 0.16 g 6.3 g 250 g...
Questions


Chemistry, 30.01.2020 10:42

History, 30.01.2020 10:42

Social Studies, 30.01.2020 10:42




Mathematics, 30.01.2020 10:42


Mathematics, 30.01.2020 10:42





History, 30.01.2020 10:42


Physics, 30.01.2020 10:42


Biology, 30.01.2020 10:42

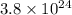
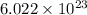 number of atoms.
number of atoms.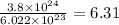 mole of argon
mole of argon