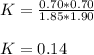Chemistry, 11.11.2020 17:50 karlacarreras152
For the following reaction at equilibrium SO3(g) + NO(g) = SO2(g) + NO2(g)It is found that [SO2] = 0.70 M and [NO] = 1.20 M. Calculate the equilibrium constant for the readction knowing that the initial concentration were [SO3] = 2.55 M and [NO] = 1.90 M.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

You know the right answer?
For the following reaction at equilibrium SO3(g) + NO(g) = SO2(g) + NO2(g)It is found that [SO2] = 0...
Questions

Mathematics, 05.11.2020 21:10



Computers and Technology, 05.11.2020 21:10

English, 05.11.2020 21:10

Mathematics, 05.11.2020 21:10

Mathematics, 05.11.2020 21:10

Mathematics, 05.11.2020 21:10

Biology, 05.11.2020 21:10


Mathematics, 05.11.2020 21:10



Spanish, 05.11.2020 21:10






Arts, 05.11.2020 21:10

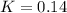
![K=\frac{[SO_2][NO_2]}{[SO_3][NO]}](/tpl/images/0887/9424/17768.png)
 is:
is: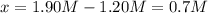
![K=\frac{x*x}{([SO_3]_0-x)([NO]_0-x)}](/tpl/images/0887/9424/71b6f.png)
![[SO_3]=2.55M-0.70M=1.85M](/tpl/images/0887/9424/debed.png)
![[NO]=1.20M](/tpl/images/0887/9424/e6bfc.png)
![[SO_2]=0.70M](/tpl/images/0887/9424/ae391.png)
![[NO_2]=0.70M](/tpl/images/0887/9424/98697.png)