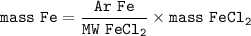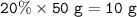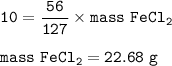Chemistry, 11.11.2020 18:30 GoAheadAndSmileToday
A mixture contains 20% iron ions by mass. What mass of iron chloride (FeCl) would
you need to provide the iron ions in 50 g of the mixture? A of Fe = 56, A of Cl = 35.5.
.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1


Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2
You know the right answer?
A mixture contains 20% iron ions by mass. What mass of iron chloride (FeCl) would
you need to provi...
Questions

Mathematics, 27.10.2020 05:00


English, 27.10.2020 05:00

Health, 27.10.2020 05:00

Biology, 27.10.2020 05:00

History, 27.10.2020 05:00

Mathematics, 27.10.2020 05:00

Mathematics, 27.10.2020 05:00

Mathematics, 27.10.2020 05:00



Mathematics, 27.10.2020 05:00

Mathematics, 27.10.2020 05:00

Mathematics, 27.10.2020 05:00


Social Studies, 27.10.2020 05:00



English, 27.10.2020 05:00

Spanish, 27.10.2020 05:00