Chemistry, 12.11.2020 05:50 F00Dislife
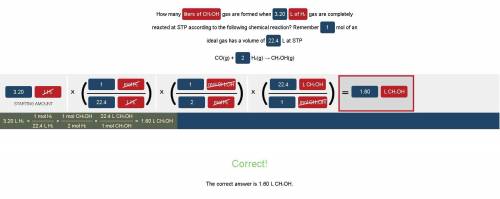
How many liters of CH3OH gas are formed when 3.20 L of H2 gas are completely reacted at STP according to the following chemical reaction?
Remember 1 mol of an ideal gas has a volume of 22.4 L at STP
CO(g) + 2 H₂(g) → CH₃OH(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1
You know the right answer?
How many liters of CH3OH gas are formed when 3.20 L of H2 gas are completely reacted at STP accordin...
Questions




Mathematics, 25.08.2020 20:01



Biology, 25.08.2020 20:01

English, 25.08.2020 20:01

Mathematics, 25.08.2020 20:01


English, 25.08.2020 20:01

Mathematics, 25.08.2020 20:01


Chemistry, 25.08.2020 20:01


Mathematics, 25.08.2020 20:01


Mathematics, 25.08.2020 20:01