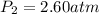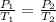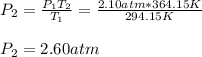Chemistry, 12.11.2020 18:30 elawnnalewis7486
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ∘C. What would the pressure be if the container was heated to 91 ∘C?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

You know the right answer?
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ∘C. What would the pressure be if...
Questions






Arts, 22.07.2019 07:30

Mathematics, 22.07.2019 07:30



Mathematics, 22.07.2019 07:30




Biology, 22.07.2019 07:30



Mathematics, 22.07.2019 07:30

Mathematics, 22.07.2019 07:30