
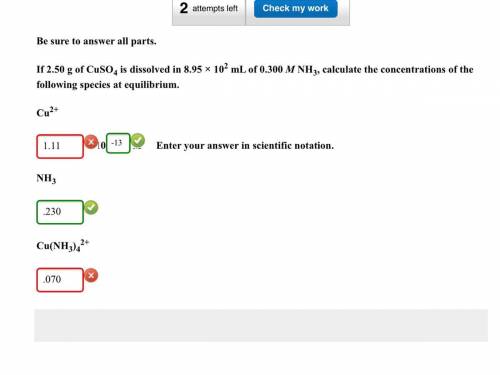
If 2.50g of CuSO4 is dissolved in 8.95 x 10^2 mL of 0.300 M NH3, calculate the concentrations of the following species at equilibrium: Cu^2+, NH3, Cu(NH3)4^2.
I tried to solve and came up with the following-
Cu^2+ = 1.1149x10^-13 (Which was all wrong except for the 10^-13)
NH3 = .230 (Which was correct)
Cu(NH3)4^2+ = 0.069720 (Which was wrong)
Can someone please show me where I am going wrong.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1


Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
If 2.50g of CuSO4 is dissolved in 8.95 x 10^2 mL of 0.300 M NH3, calculate the concentrations of the...
Questions

English, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01


Mathematics, 16.10.2020 21:01

Physics, 16.10.2020 21:01

Chemistry, 16.10.2020 21:01


Social Studies, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01

History, 16.10.2020 21:01

English, 16.10.2020 21:01


Mathematics, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01

Spanish, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01




