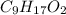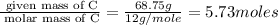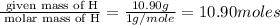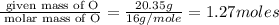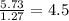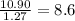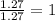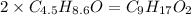Chemistry, 10.10.2019 22:50 tabbydory3366
Determine the number of atoms of each element in the empirical formula of a compound with the following composition:
68.75 percent c, 10.90 percent h, 20.35 percent o.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
Determine the number of atoms of each element in the empirical formula of a compound with the follow...
Questions




Mathematics, 17.11.2020 04:20

Geography, 17.11.2020 04:20

Mathematics, 17.11.2020 04:20

Mathematics, 17.11.2020 04:20

Mathematics, 17.11.2020 04:20

Mathematics, 17.11.2020 04:20


Physics, 17.11.2020 04:20

Mathematics, 17.11.2020 04:20

History, 17.11.2020 04:20





Mathematics, 17.11.2020 04:20

Mathematics, 17.11.2020 04:20