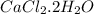Chemistry, 29.08.2019 07:00 mimireds5419
Anhydrous cacl2 is used as a desiccant in desiccators, i. e. it removes water from the air within the desiccators and forms a hydrated salt. a 16.43g mass of anhydrous cacl2 weighed 21.75g after being left in desiccators for several months. what is the formula of the hydrated cacl2?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:50
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
Anhydrous cacl2 is used as a desiccant in desiccators, i. e. it removes water from the air within th...
Questions

Mathematics, 08.04.2021 03:20

Mathematics, 08.04.2021 03:20



Mathematics, 08.04.2021 03:20



Physics, 08.04.2021 03:20


Mathematics, 08.04.2021 03:20

Chemistry, 08.04.2021 03:20





Mathematics, 08.04.2021 03:20


Mathematics, 08.04.2021 03:30

Mathematics, 08.04.2021 03:30