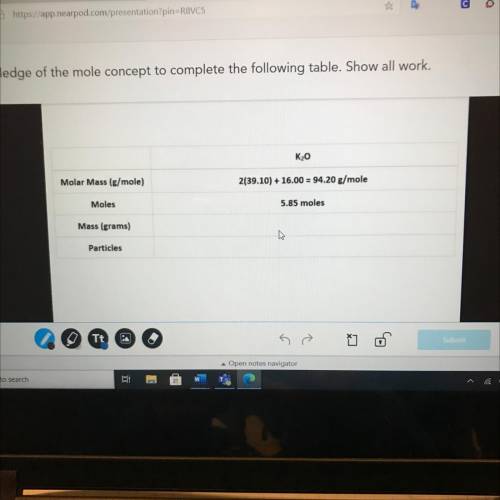
K20
Molar Mass (g/mole)
2(39.10) + 16.00 = 94.20 g/mole
Moles
5.85 moles
Ma...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

You know the right answer?
Questions




History, 19.07.2019 19:00

History, 19.07.2019 19:00



Mathematics, 19.07.2019 19:00

Mathematics, 19.07.2019 19:00


Biology, 19.07.2019 19:00

Mathematics, 19.07.2019 19:00

Mathematics, 19.07.2019 19:00

Mathematics, 19.07.2019 19:00




Spanish, 19.07.2019 19:00

English, 19.07.2019 19:00

Social Studies, 19.07.2019 19:00