Chemistry, 13.11.2020 06:20 msmojangles
Assume a density of water of 1.00 g/mL, and calculate the mass of water in the solution

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
Assume a density of water of 1.00 g/mL, and calculate the mass of water in the solution...
Questions

Mathematics, 24.08.2021 07:30

Mathematics, 24.08.2021 07:30

Mathematics, 24.08.2021 07:30

Mathematics, 24.08.2021 07:30

Physics, 24.08.2021 07:30

Mathematics, 24.08.2021 07:30

Physics, 24.08.2021 07:30


English, 24.08.2021 07:30

Mathematics, 24.08.2021 07:30

English, 24.08.2021 07:30

Mathematics, 24.08.2021 07:30

Mathematics, 24.08.2021 07:30

History, 24.08.2021 07:30

Mathematics, 24.08.2021 07:30


Mathematics, 24.08.2021 07:30

English, 24.08.2021 07:30

History, 24.08.2021 07:30

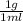 so from this equation, you will get 1 g and you can to SI to be
so from this equation, you will get 1 g and you can to SI to be 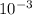 kg
kg