Calculate the number of moles in 3.00 × 108 L of 0.0400 mM CrO42–.
...

Chemistry, 13.11.2020 07:40 whitethunder05
Calculate the number of moles in 3.00 × 108 L of 0.0400 mM CrO42–.
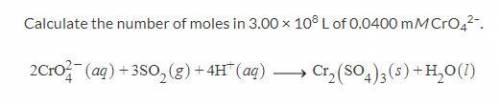

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
Questions







Computers and Technology, 10.03.2020 22:41


Mathematics, 10.03.2020 22:41










History, 10.03.2020 22:42



