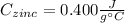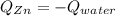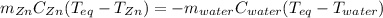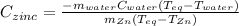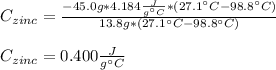Chemistry, 13.11.2020 20:00 missjohnson4449
A 13.8 g of zinc is heated to 98.8 c in boiling water and then dropped onto a beaker containing 45.0 g of water at 25.o °C .when the water and metal come to thermal equilibrium the temperature is 27.1°C .what is the specific heat capacity

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2
You know the right answer?
A 13.8 g of zinc is heated to 98.8 c in boiling water and then dropped onto a beaker containing 45.0...
Questions



Mathematics, 18.11.2020 23:50

Mathematics, 18.11.2020 23:50

English, 18.11.2020 23:50


Biology, 18.11.2020 23:50

Biology, 18.11.2020 23:50


Mathematics, 18.11.2020 23:50

Mathematics, 18.11.2020 23:50


Mathematics, 18.11.2020 23:50

Biology, 18.11.2020 23:50




Mathematics, 18.11.2020 23:50


English, 18.11.2020 23:50