
Chemistry, 13.11.2020 20:40 amayarayne5
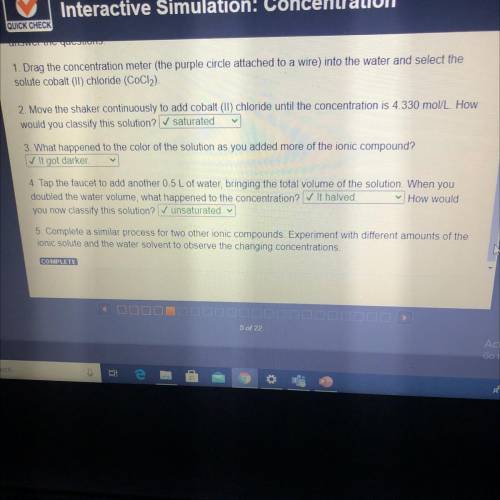
1. Drag the concentration meter (the purple circle attached to a wire) into the water and select the
solute cobalt (II) chloride (CoCl2).
2. Move the shaker continuously to add cobalt (II) chloride until the concentration is 4.330 mol/L. How
would you classify this solution? ✓ saturated
3. What happened to the color of the solution as you added more of the ionic compound?
✓ It got darker
4. Tap the faucet to add another 05 L of water, bringing the total volume of the solution. When you
doubled the water volume, what happened to the concentration? It halved.
How would
you now classify this solution? ✓ unsaturated
5. Complete a similar process for two other ionic compounds Experiment with different amounts of the
ionic solute and the water solvent to observe the changing concentrations.
COMPLETE

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
1. Drag the concentration meter (the purple circle attached to a wire) into the water and select the...
Questions


Chemistry, 05.10.2019 19:00



Mathematics, 05.10.2019 19:00


Mathematics, 05.10.2019 19:00


Social Studies, 05.10.2019 19:00



Physics, 05.10.2019 19:00




Mathematics, 05.10.2019 19:00


English, 05.10.2019 19:00

History, 05.10.2019 19:00



